Iron Treatment Shows Promise in Children with RLS or PLMD
Tuesday, April 26, 2022April 26, 2022 Iron Treatment Shows Promise in Children with RLS or PLMD By Karla Dzienkowski, RN, BSN, Executive Director, RLS Foundat...
Iron Treatment Shows Promise in Children with RLS or PLMD
By Karla Dzienkowski, RN, BSN, Executive Director, RLS Foundation
Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD) are two neurological conditions commonly treated with oral iron in children. Some children do not tolerate or respond sufficiently to oral iron, and there is not sufficient data to support the efficacy of intravenous (IV) iron in this patient group. This is an important issue, because RLS that is not well managed can have adverse effects on sleep quality and quantity, emotional health, daytime functioning, and quality of life.
RLS diagnostic criteria for children are:
- Urge to move
- Rest induced
- Gets better with movement
- Evening or nighttime worsening
- Sensations not caused by another behavioral or medical condition (may or may not be present)
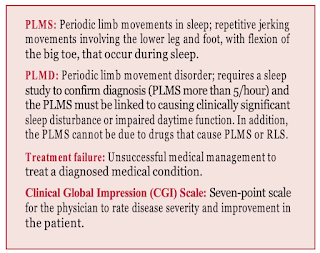
An RLS diagnosis in children is based on the clinical assessment of reported symptoms and does not require documentation of periodic limb movements in sleep (PLMS); however, PLMS (more than 5/hour) do support a diagnosis of RLS and are present in 60% to 70% of children with RLS.
Researchers have hypothesized a relationship between low brain iron stores in both RLS and PLMD. Brain imaging studies have confirmed the presence of low brain iron levels in adults with RLS. In addition, symptom improvement following iron administration in children with RLS and PLMD is reported in medical publications.
An article in the November 2021 issue of Sleep Medicine titled “Clinical efficacy and safety of intravenous ferric carboxymaltose treatment of pediatric restless legs syndrome and periodic limb movement disorder” evaluates the single administration of IV iron in 39 children with a diagnosis of RLS or PLMD confirmed by two pediatric sleep specialists. The participants in the study included 29 children with RLS (14 boys, 15 girls) and 10 with PLMD (all boys), ages 2–17 years. Children in this cohort were selected using a retrospective chart review; they were referred for iron treatment from June 2019 to December 2020. All children selected to participate in the study had previously been prescribed oral iron but were categorized as “treatment failures” due to untoward side effects (e.g., constipation, vomiting, tooth discoloration) or failure to increase serum ferritin by at least 4 mcg/L after three months of taking an oral iron supplement, in conjunction with a lack of symptom improvement. It is important to note that none of the children in the study had another sleep disorder or a known problem absorbing nutrients from food (malabsorption syndrome).
Seattle Children’s Hospital was the only site selected for this study. The hospital has a dedicated iron infusion clinic for children supervised by a pediatric sleep specialist. The children were carefully monitored throughout the IV iron infusion process, including monitoring for the occurrence of any adverse reactions. All 39 children received a complete medical evaluation. A full iron panel (serum ferritin, serum iron, total iron-binding capacity, transferrin) and Clinical Global Impression (CGI) Scale score was obtained at baseline and eight weeks post-infusion with ferric carboxymaltose.
The study found:
- Pre-infusion CGI symptom severity for the group showed moderate illness. Post-infusion reports revealed “much improved” to “very much improved” CGI scores following one-time administration of IV ferric carboxymaltose.
- Mean ferritin levels for the group rose from 14.6 mcg/L to 112.4 mcg/L.
- Children with PLMD, compared to those with RLS, had similar improvements in symptoms and iron tests.
- Seven study participants experienced mild side effects to IV iron treatment.
In summary, in children with RLS and PLMD, iron is a common treatment consideration. For children who experience a poor response or adverse effects to oral iron therapy, IV iron is a promising new treatment alternative. Future research using randomized, controlled trials is needed to evaluate further the benefits of IV iron therapy in children.
DelRosso LM, Ferri R, Chen ML, Kapoor V, Allen RP, Mogavero MP, et al. Clinical efficacy and safety of intravenous ferric carboxymaltose treatment of pediatric restless legs syndrome and periodic limb movement disorder. Sleep Medicine. 2021;87:114-8